Hey aquarium enthusiasts! Ever wondered just how tough these tiny creatures called brine shrimp really are? Well, I’m gonna blow your mind with some fascinating facts about their incredible ability to survive in super salty environments. As someone who’s been keeping aquariums for years, I’ve always been amazed by these little survivors
Quick Answer
Brine shrimp can withstand salt concentrations ranging from regular seawater (35 g/L) all the way up to near-saturation levels of 250 g/L! Their optimal range is between 40-80 g/L, but they’re incredibly adaptable
Why Are Brine Shrimp So Special?
These tiny extremophiles (that’s what we call organisms that love extreme conditions) have some pretty impressive superpowers when it comes to dealing with salt. Here’s what makes them so special:
- They can live in waters ranging from regular seawater (2.9-3.5%) to super salty environments like the Great Salt Lake (25-35%)
- They’ve got specialized gills that help them deal with salt
- They can pump excess ions out of their bodies
- They can survive in temperatures from 6°C to 37°C
Natural Habitats: Where Do These Salt-Lovers Live?
You’ll find these tough little creatures in some pretty intense places
- Great Salt Lake (Utah, USA)
- Salt lakes in the Atacama Desert
- Mediterranean salt works
- Coastal saltworks worldwide
- Salt swamps near coastlines
But here’s something interesting – you won’t find them in the ocean! Not because it’s too salty (it’s actually not salty enough), but because there are too many predators there. Pretty smart of them, right?
How Do They Handle All That Salt?
Brine shrimp have developed some pretty neat tricks to deal with high salt concentrations:
-
Super-Powered Osmoregulation
- They can actively pump ions out of their bodies
- Their gills help them absorb and excrete ions as needed
- They produce concentrated urine through special glands
-
Cellular Adaptations
- Their cells can handle salt levels twice that of seawater
- They maintain proper internal pressure even when external conditions go crazy
-
Emergency Survival Mode
- They can form cysts (like little protective shells) when conditions get too extreme
- These cysts can survive for up to 50 years!
Optimal Salt Conditions for Keeping Brine Shrimp
If you’re thinking about raising brine shrimp (maybe as fish food or even pets), here’s what you need to know:
| Condition | Measurement |
|---|---|
| Minimum Salt Level | 25 g/L |
| Optimal Range | 30-35% |
| Maximum Tolerance | Up to 250 g/L |
| Recommended for Hatching | 30-35 g/L |
Pro Tip: For each gallon of water, use about 7-8 tablespoons of aquarium salt. Don’t use table salt – it’s got iodine that can harm your shrimp!
Why Should You Care About Brine Shrimp?
These little guys are pretty important for several reasons:
- They make excellent live food for aquarium fish
- They’re great for toxicity testing
- They’re used in education (remember Sea Monkeys?)
- They’re easy to breed and maintain
- They cost only about $7 per pound
Common Mistakes to Avoid
When keeping brine shrimp, watch out for these rookie errors:
- Using table salt instead of aquarium salt
- Not maintaining proper temperature (aim for around 25°C)
- Forgetting to provide enough oxygen
- Overlooking water quality maintenance
Final Thoughts
I gotta say, brine shrimp are some of the most fascinating creatures in the aquarium hobby. Their ability to survive in such extreme conditions is just mind-blowing! Whether you’re using them as fish food or keeping them as pets, understanding their salt tolerance is key to success.
Remember, while they can handle super high salt concentrations, you don’t need to push them to their limits. Stick to the optimal range of 30-35% salinity, and your brine shrimp will thrive!
Would you like me to explain any part of this in more detail? Drop a comment below!
#aquariumlife #brineshrimp #extremophiles #aquariumcare
Artemia: A Survival Machine
The suite of responses Artemia has evolved to cope with the harsh conditions imposed by salty lakes is schematically shown in Figure 1. The most visible response is the ability of females of all species, including parthenegenetic forms, to switch reproductive mode, or offspring quality (Gajardo and Beardmore, 1989), depending on whether the environment is perceived as stable, generally the case of permanent lakes, or stressful, like seasonal lakes that dry-out. Lack of food, low oxygen tension, extremely high or low salinity and temperature (even lack of available mates) are stressors as well. In the first case females produce predominantly offspring in the form of free-swimming nauplii (ovoviviparity) or as cysts (oviparity). We subscribe to the statement of Clegg and Trotman (2002) that cysts are “the most resistant of all animal life history stages to environmental stress,” at least based on the experience with A. franciscana, the most studied species, whilst motile stages (nauplii and other larval stages and adults) are “the best osmoregulators in the animal kingdom.” Both life history stages are schematically treated in the “organism” compartment of Figure 2, but also because they have different adaptive repertoires as they are programmed to face different environmental conditions as indicated above. Salty lakes modulate also the population and species compartment (Figure 2). With regard to organisms, there are 324 protein-coding genes associated with diapause and post-diapause influencing cell components, molecular functions, such as antioxidant activity that protect desiccated cysts from molecular oxygen, and biological processes like development, growth, response to stimulus and interaction between individuals, as reported by Chen et al. (2009). Some of these proteins are stored in cysts and utilized after their reactivation, or are involved in anti-desiccation and diapause mechanisms. Just to mention a few of them: cathepsin-like cysteine proteinase involved in yolk utilization in embryos, proteases, protease inhibitors, and chaperones (p26, Hsp70) that prevent protein aggregation. In stressed cells, Hsp70 and p26 move to the nucleus to stabilize matrix proteins. Maintaining cell membrane fluidity (or prevent it from vitrification) in the encysted embryo by means of the sugar trehalose (Clegg and Trotman, 2002) involves critical fractal physiological processes that protect from the destructive effects of dehydration, chiefly through its ability to serve as a substitute for structural water associated with the membrane surface. Likewise, adaptation of nauplii to brine is another magnificent process that began to be studied long ago (Croghan, 1958a,b). It is achieved by maintaining hemolymph at concentrations and compositions considerably lower and qualitatively different from the external hyperosmotic medium and this requires retaining or taking up water, while excess ions are eliminated to the medium against huge concentration gradients (reviewed by Clegg and Trotman, 2002). The key component in this ion-transporting process in larval stages is the salt gland and its enzyme Na, K-ATPase that first appears in emerged embryos, increases dramatically as the larvae hatch and pass through the first instar epithelia of animals in general (Conte, 1984). High salinity imposes the extra challenge of reduced dissolved oxygen, hence efficient oxygen uptake (Dwivedi et al., 1987) becomes vital to avoid impairment of critical functions such as swimming and feeding. As salinity increases and oxygen tension diminishes, hemoglobin synthesis increases. However, respiratory rates differ between nauplii and adults, and between females and males, a process mediated by three hemoglobin types, resulting from the permutation of subunits α and β, differing in their oxygen affinity, and so affecting the physiological response to oxygen availability (Clegg and Trotman, 2002).
With regard to the population and species compartment (Figure 2), the most striking phenomenon here is that overall high genetic diversity (see Gajardo et al., 2002; Baxevanis et al., 2006; Muñoz et al., 2010) of the species is heterogeneously distributed over the different component populations. In other words, the species gene pool is distributed over different safety baskets along the distribution range. Such ecological divergence is the mode of speciation evolved by the group as proposed by Abreu-Grobois and Beardmore (1982) and Abreu-Grobois (1987), see also Gajardo et al. (2002). Ecologically adapted populations retain local adaptations in a process modulated by differential selective pressures of each environment. Local populations are caused by geographic isolation of the island-like nature (Gajardo et al., 2002) of salty lakes that restrict gene flow mediated by water birds, the natural cyst dispersers together with wind. Cycles of extinction and re-colonization, expansion and contraction, observed in seasonal lakes or ponds that dry-out, are also significant drivers of genetic differentiation. Such ecological specificity has trade-offs, the price paid is isolation and solitude; in other words, Artemia is prevented from interaction with other species, for good or bad. There would be restrictions between populations as well, as reported by Rode et al. (2011). These authors performed crosses between females and males hatched from cysts collected at different times, and found that females survived better and had longer inter brood intervals when mated with their contemporary males compared to when they were mated with males from the future or the past. A similar example in Drosophila showed that females adapted to specific ecological conditions, and therefore with a particular bacterial composition in the gut, mate preferentially with males with the same similar gut composition (Sharon et al., 2010).
How Many Artemia Species are There?
Gajardo et al. (2002, p. 226, Table 1) listed some of the attributes that make Artemia a good model to study adaptation and speciation. This organism is also considered a paradigmatic species in understanding the evolutionary biology of arthropods and related groups (Marco et al., 1991; Chen et al., 2009). A look at the existing Artemia species and their distribution shows three striking facts in which the species (life history and genetic background) and the environment are tightly linked:
- (1)there are six sexual species, a relatively low number, most of them geographically restricted to salty lakes in specific regions in Eurasia (regional endemism) at, or close to, the Mediterranean area where Artemia species diverged from the ancestral species some 80 million years ago (Baxevanis et al., 2006). These are the so-called Old World species: Artemia urmiana (Günther, 1890), Lake Urmi, Iran, where there are also parthenogenetic populations. But also the species would be present in Ukraine. Artemia tibetiana (Abatzopoulos et al., 1998, 2003), Tibetan Plateau. Artemia sinica (Cai, 1989), PR of China, and Mongolia. Artemia salina (Leach, 1819), Mediterranean Basin. Artemia sp. Kazakhstan (Pilla and Beardmore, 1994), since only a single sample from Kazakhstan was studied the specific status is still under discussion.
- (2)There are parthenogenetic populations as well, as indicated above, and these originated in multiple events in Central Asia either from A. urmiana or the Kazakhstan population (Muñoz et al., 2010), and probably from all of the sexual species indicated above (Baxevanis et al., 2006). Parthenogens would have migrated to the Mediterranean Basin where a dramatic salinity increase and habitat subdivision (Abreu-Grobois, 1987) would have facilitated their expansion, very likely in the form of cysts (Muñoz et al., 2010). The estimated time for the appearance of asexual forms varies according to the genetic markers and the methodology of analysis considered: three MYA (Baxevanis et al., 2006), 40 MYA (Badaracco et al., 1987) or very recently (Muñoz et al., 2010). The importance of unusual habitats (different from those occupied by parental sexual species) seems essential for the generation and maintenance of a parthenogenetic mode (Muñoz et al., 2010), and also is evident from the fact that parthenogenetic forms tend to co-exist, and even displace sexuals under special conditions (Amat et al., 2005).
- (3)Two species are found in the Americas (New World species): A. franciscana and A. persimilis, the former widely divergent from the Old World species (Gajardo et al., 2002), though some similarity with all Asian species and parthenogens has been found by comparing mitochondrial ITS1 sequences and 16 S RFLP markers (Baxevanis et al., 2006). A. persimilis is closer to Old World species, in fact it is the most widely divergent of all Artemia species as determined by the use of different genetic markers (Gajardo et al., 2002; Baxevanis et al., 2006; Muñoz et al., 2008; Kappas et al., 2009), and is restricted to southern latitudes in South America, particularly Argentina and Chile. Although bar coding and other genetic markers are useful for assessing genetic distance between species, data of this nature need to be viewed with some caution, taking into account recent observations showing that not all genes are equal in the light of evolution. In other words, evolutionarily relevant mutations tend to accumulate in hotspot genes and at specific locations within genes (Stern and Orgogozo, 2009). Having said that, we note that A. franciscana has a very distinctive feature separating it from all other species. This feature is its chromo centre number, i.e., highly repetitive heterochromatin, which is known to play a role in chromosome segregation and reorganization, nuclear and cellular organization, and regulation of gene expression (MacGregor and Sessions, 1986; see Hayden, 2010, for humans). Thus, non-coding regions matter significantly, and because of that it is highly relevant to note that this trait varies significantly with latitude in A. franciscana (Gajardo et al., 2001), the most widely distributed species (also found invading non-native areas like the Mediterranean, Amat et al., 2005). Due to its repetitive nature, heterochromatin is prone to expansions and contractions (Parraguez et al., 2009, and references therein), thus representing an opportunity for rapid genomic change.
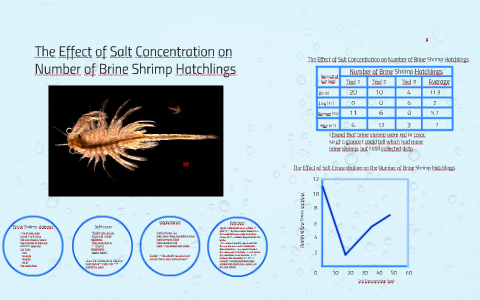
Brine Shrimp Hatch — A Qualitative Observation in Varying Salt Concentrations
FAQ
What salinity can brine shrimp live in?
The average adult male brine shrimp is 0.3–0.4 inches long, and the average female is 0.4–0.5 inches long. They can survive in water with salinities ranging from 30–330 g/l (3% to 33% salinity).
Can water be too salty for brine shrimp?
Comments Section From “Sea Monkeys & Brine Shrimp: A Keepers Handbook”: “Whilst brine shrimp can withstand a wide range of salinity levels (25%-250%) that doesn’t mean that they like or are able to cope with big variations or sudden drastic changes in salinity levels. The best range for them is between 30-35 ppt.
Can you use any salt for brine shrimp?
This equates to around 1.018 specific gravity as measured with a hydrometer. If you lack a hydrometer, this salinity can be achieved by dissolving approximately 1 and 2/3 tablespoons of salt in one quart (roughly, one liter) of water. Be sure to use non iodized salt.
What are brine shrimp sensitive to?
Like many water creatures, brine shrimp have evolved to be most sensitive to blue light, the color of light that’s best transmitted in water.
